Discover
Visualize rapid, validated insights through real-world data.
Datavant Completes Acquisition of Aetion. Learn more →

JMDC, a leading Japanese health big data firm, and Aetion® recently announced a partnership that will see the organizations working together to improve healthcare outcomes for patients globally through the combination of real-world data (RWD) and innovative evidence generation technology.
This partnership comes at a crucial time when RWD and real-world evidence (RWE) have gained broader regulatory and commercial acceptability, yet there is still much work to be done in order to meet a number of challenges that face the healthcare industry—including a rapidly aging population, disparities in medical care, lifestyle-related diseases, and labor shortages.
To further explore how this partnership seeks to address these challenges, Aetion’s SVP, Real-World Data & Delivery Operations, Wendy Turenne, sat down with Makoto Kano, Executive Officer, Pharmaceutical Division at JMDC to learn about the value of big data to society, the RWD landscape in Japan, and how the partnership with Aetion will enhance Japanese RWD on a global scale.
JMDC focuses on a future that affords everyone a healthy and prosperous life. Could you tell us more about what this means?
JMDC was founded in Japan in 2002. Since then, our organization has been a pioneering presence in RWD and evidence generation.
In recent years, the sense of urgency around skyrocketing medical expenses – often referred to as the “2025 problem” – has gained traction. We at JMDC have been partnering with a wide range of organizations and databases to address this challenge. Our goal is to use data to improve the “sustainability of medical expenses” and positively impact global health.
At the grassroots level, JMDC has been working to realize our vision by building an ecosystem. This ecosystem offers services to healthcare-related entities such as physicians, patients, health insurance associations, medical institutions, local governments, pharmaceutical companies, and life insurance companies that utilize data and Information and Communication Technologies (ICT). These services are geared towards enhancing care and streamlining medical processes.
Through these data-centric services, JMDC aims to contribute to the soundness of medical expenses by fostering the creation of innovative treatment methods and establishing their evidence. We believe in a future where everyone can pursue their dreams and lead healthy lives.
How are you using “Big Data” to return value to society?
We are returning value to society by using data to support various stakeholder activities. Here are a few examples of the many ways we’re making an impact:
Tell us about JMDC’s data collection resources. How do you curate the data, and what kinds of data do you make available to customers?
JMDC covers nearly all available medical big data in Japan. The data sources include company-operated Payers, local governments, medical institutions of various sizes and forms of inpatient functions, and pharmacies that process prescription data. All databases provided for external use are anonymized. The characteristics of each database are as shown in the following diagram.
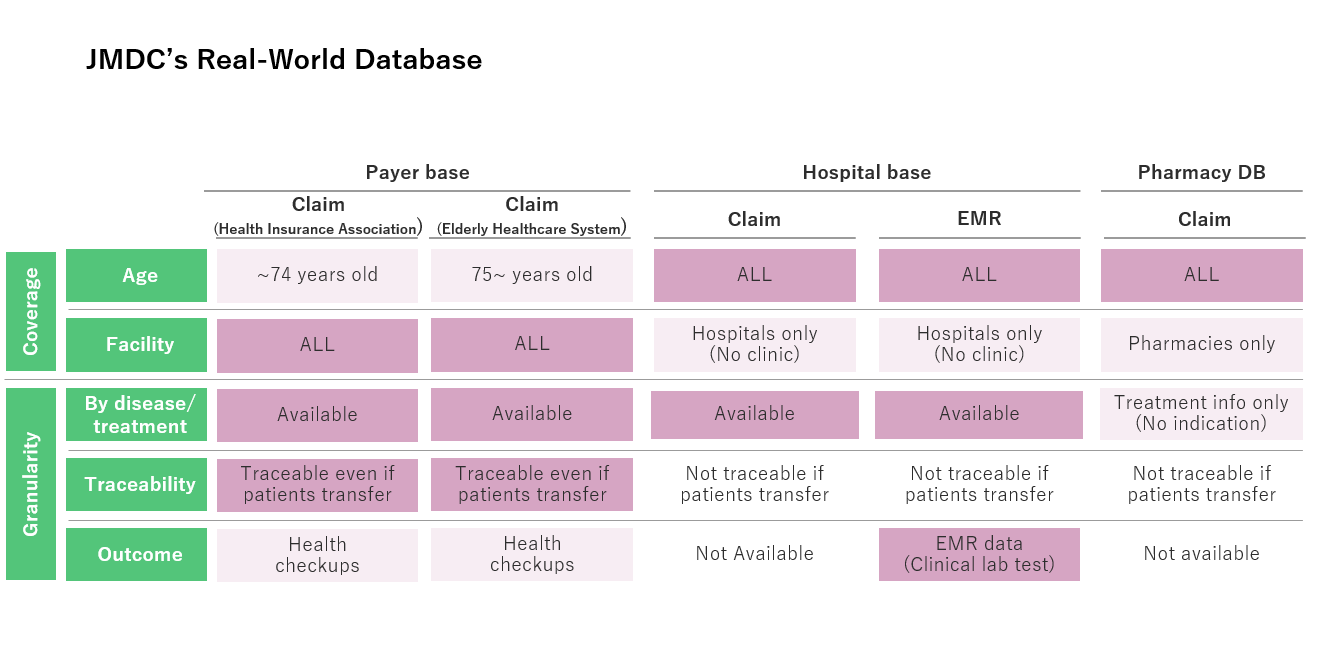
The data sources encompass more than just claims data. They include foundational ledger data that reveals information like enrollment histories for insurance, electronic medical record (EMR) data from medical institutions (primarily clinical test values), health examination results received under insurance subsidies, Disease Procedure Combination (DPC) survey data recording disease severity of hospitalized patients, admission pathways, daily nursing requirements, and more.
By utilizing these databases, it becomes possible to observe patient populations across all age groups and various types of medical facilities. For instance, it enables the elucidation of patient journeys for specific diseases, detailing the chronological and cross-facility aspects of treatment, outcomes, and more.
Clients primarily comprise pharmaceutical and medical device companies, who utilize the data extensively – from R&D to Sales and marketing and Post-Market Surveillance (PMS). Notably, there have been more than 600 studies that use JMDC data as evidence, and more than 15 major pharmaceutical companies have licensed the full dataset.
In the United States and Europe, regulatory agencies have begun issuing guidance on accepting real-world data for decision-making. What is the landscape in Japan?
In Japan, efforts and considerations are being led by the government to utilize RWD for decision-making. For pharmaceutical companies, the current and mid-term utilization of RWD is expected to continue to be centered around representative commercial databases like JMDC.
However, several barriers must be overcome to ensure high-quality decision-making. These include challenges in the inefficiency of industry-academia collaboration in patient registries, time lag issues with National Databases, and the diverse system specifications of electronic medical records.
For pharmaceutical companies, various policies are being implemented to utilize RWD, particularly in the context of manufacturing and marketing authorization applications, as well as re-evaluation applications.
The current methods for utilizing RWD in approval applications include:
The utilization of RWD is expected to advance, not just in approval applications but for other purposes.
Congratulations on your recent acquisition of RWD Co. How has the integration progressed?
Thank you! Currently, we are working on integrating JMDC and RWD Co,’s databases. Once the integration is complete, a highly representative database will emerge, covering up to 10% of all medical facilities with inpatient capabilities in Japan, including non-DPC (non-acute) hospitals.
With access to RWD Co.’s database, we can now leverage electronic medical record data (diagnoses, clinical test values) in addition to the existing claims information. For instance, electronic medical records capture information that might not be recorded in claims, such as conditions without insurance-covered medications and ongoing observations (e.g., self-paid visits during pregnancy, COVID-19 cases, oncology field, etc.). This enables us to understand the patient journey in greater detail, compared to using claims data alone, and conduct more precise market size estimations.
The integration with RWD Co. has also expanded the volume and variety of clinical test value data available. Leveraging the connection between RWD and medical institutions, we are also working on enabling clients to acquire new data on demand. While default clinical test values mainly include blood and urine tests, we’ve recently incorporated electrocardiogram data. Moving forward, we aim to propose solutions that combine the databases of both companies.
What other technologies are you currently exploring or interested in?
We are actively working on obtaining data beyond claims, including clinical test values from electronic medical records, non-structured data, open data sources, personal health records via patient platforms, and a range of medical information exchanged within our proprietary physician panels. We’re gradually broadening the scope of data utilization using these sources and are prepared to offer solutions addressing challenges across various scenes within pharmaceutical companies.
How do you see the Aetion®-JMDC partnership creating value for the healthcare industry and patients?
Especially at a global level, we feel that information about patients in Japan is lacking. Therefore, through the Aetion-JMDC partnership, we place great importance on enhancing the global presence of the Japanese market. By combining our informative and extensive data with Aetion’s robust analytical capabilities, we can elevate the resolution of insights concerning the Japanese market and patients for various stakeholders.
This, in turn, can contribute to swift clinical trials, appropriate marketing strategies, safety monitoring activities, and more. Ultimately, this aligns with our vision of creating a world where everyone can afford a healthy and prosperous life.
Tell us more about what partnership means to you and why you selected Aetion® as a partner to help move RWE solutions forward.
As mentioned earlier, Aetion’s extensive network within the pharmaceutical industry can enhance the presence of Japanese RWD on a global scale. Furthermore, the strong reliability of Aetion’s technology is another reason for choosing Aetion, given its track record in Regulatory Science Research at the FDA and other applications.
RWD comes with distinct characteristics and limitations depending on the data sources and collection methods. To generate real-world evidence, it’s essential to have mechanisms to handle each database appropriately, along with sound analytical logic. We consider Aetion’s platform to provide these necessary elements.
As we look to the future, where does JMDC hope to be in the next five years?
Over the next five years, we will focus on strengthening our assets and advancing the societal implementation of data to drive societal transformation. Our specific goals include: